Pharmaceutical research sits at the intersection of science, safety, and regulation. It takes years of hard work, testing, and double-checking to get a new treatment ready. Even small problems or missing paperwork can cause big delays and cost a lot of money. Plus, it can really affect patients who are waiting for those new medicines.
When research leaders evaluate the best project management tools, they find that their needs go far beyond project tracking. Pharma research demands platforms that centralize communication, standardize documentation, manage regulatory workflows, and ensure approvals are accountable. Lark provides this structure, enabling research teams to move quickly while staying compliant every step of the way.
Documenting trials and protocols with Lark Docs
Medical studies create a mountain of paperwork, like trial plans, consent forms, safety reports, and science papers. If you’re emailing these files around or saving them in multiple locations, it’s easy to lose the most up-to-date version. Lark Docs solves this by giving your team a single spot to write, edit, and review documents together
This means that scientists, compliance folks, and outside reviewers can easily update clinical trial plans as a team without getting confused. Every change is tracked, and permissions keep sensitive files secure. Researchers don’t have to guess which draft is the newest. They can work with confidence, saving time and lowering the chance of compliance problems. This consistency is really important for long, complex trials.
Tracking deadlines with Lark Calendar
In the world of pharmaceutical research, timelines are super important. From getting approval from ethics committees to hitting data collection goals, every step has to follow the rules. If you miss a deadline, it can cause delays in approvals or force you to redo submissions, costing a lot of money. Lark Calendar helps teams keep track of these important schedules by laying out trial phases, compliance checks, and what needs to be reported.
With shared calendars, everyone can see what’s coming up, and permissions make sure sensitive info stays private. Tasks made right in Lark show up in the Lark Calendar automatically. This means smaller deadlines, like collecting data or doing follow-up analysis, stay on track with bigger goals. For example, when getting ready to submit something to regulators, you can create a task accordingly, which will be synced to Calendar shown in Calendar when drafts are due. This gives researchers and administrators a clear plan that everyone can share.
Coordinating trial teams with Lark Messenger
Clinical trials usually involve many locations, global teams, and outside helpers. When communication lags, data can become messy, and plans can go off track. Lark Messenger makes it easy for everyone involved—staff, researchers, and managers—to chat in real time. You can set up topic groups for specific trials, parts of a trial, or even where the study is happening, which keeps everything neat.
Threaded replies help keep talks about things like getting patients, watching out for safety, or doing lab tests on point and easy to find later. Things like permission slips or lab guides can be right there in Lark Messenger, connected to the chat. For example, if a lab in one place finds something weird, they can share it right away with researchers and compliance folks everywhere else. This speeds up decisions, lowers risks, and keeps the trial solid.
Ensuring accountability with Lark Approval
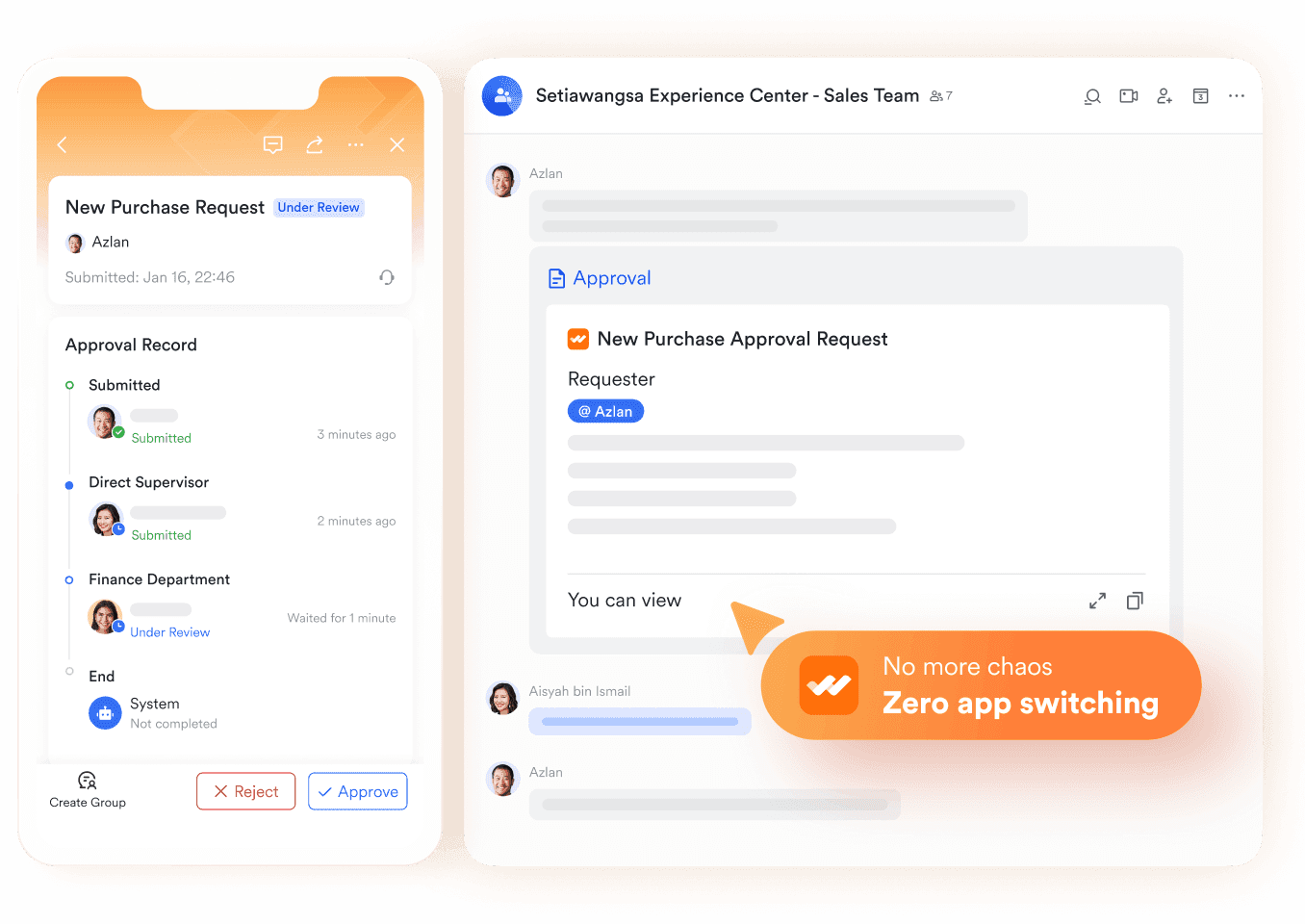
Few industries face stricter approval requirements than pharma. Ethics committee reviews, protocol amendments, trial budget adjustments, and procurement for lab equipment all require structured sign-offs. Managing these through email or paper slows progress and increases the chance of errors. Lark Approval creates a digital workflow where every request is submitted, reviewed, and logged in a transparent system.
This is where Lark demonstrates its role as business process management software. A trial coordinator requesting approval for an amended patient recruitment process can submit it digitally, with decision makers reviewing and recording the outcome in minutes rather than days. Every approval leaves an auditable trail, ensuring compliance requirements are met while keeping research timelines intact. Patients benefit because studies advance without unnecessary administrative delays.
Structuring research workflows with Lark Base
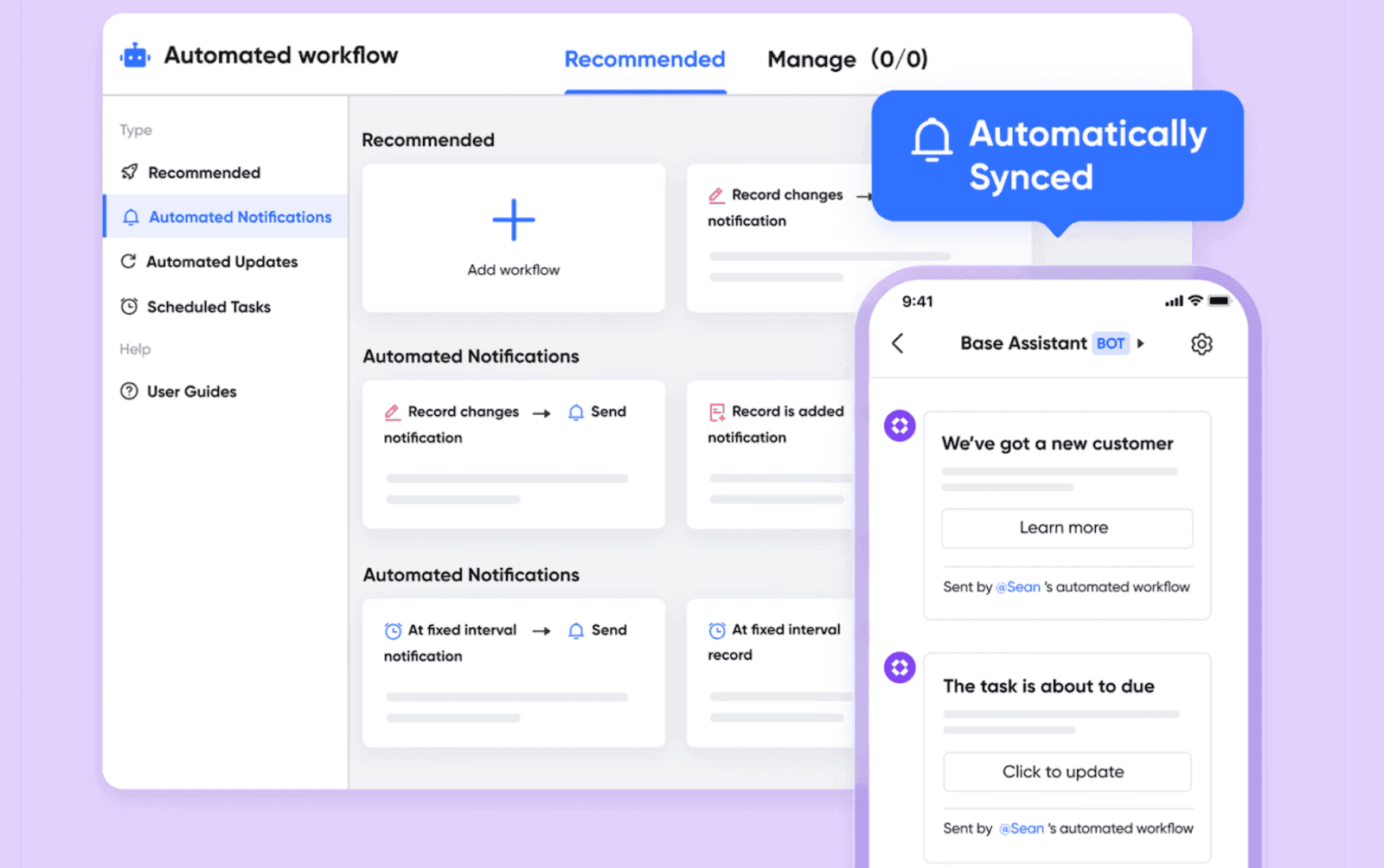
Every trial that goes well depends on a mix of data, tasks, and rules. Spreadsheets can only do so much before they get messy. As a smart CRM app, Lark offers Base as its solution. Lark Base gives you a place to create trial plans that fit what each study needs. You can change tables to keep track of people in the trial, watch safety checks, and handle lab results. Views let you sort by location, stage, or rule.
Automated actions make things even more reliable by sending alerts for safety report deadlines or telling managers when they’re close to reaching enrollment goals. think about it, a vaccine trial in different countries can follow patient sign-ups, lab test progress, and legal steps in Lark Base. With updates as they happen, managers can see what’s going on without giving workers too much information. This makes sure the research moves ahead safely and as expected.
Documenting and sharing outcomes with Lark Meetings

In drug research, you’re always going to meetings about investigations, safety, and rules. The problem is, what comes out of these meetings often ends up scattered all over the place – in notebooks or on people’s computers. Lark Meetings can fix that by making sure every meeting has clear, written results.
When it comes to the meeting, Lark offers high-quality audio and clear video sharing. To break language barriers, it supports 24 language translation and subtitles. After clicking AI Summary, notes will be taken during calls and get saved in Lark Docs afterwards. Let’s say you just had a call about global safety. Lark Meetings lets you write down who needs to do what and assigns those tasks, so nothing falls through the cracks. Regulators, trial leaders, and site staff all get to see the same decisions. Instead of vague promises, meetings end with real steps people can take. This keeps trials on track, follows the rules, cuts down on delays, and makes regulators trust research companies more.
Conclusion
When research and following the rules go together, drug studies get better. Lark Docs, Calendar, Messenger, Approval, Base, and Meetings can give research teams a place to keep files easy to read, get quick okay’s, and talk fast. When studies stay on time, the chance of breaking rules goes down, and data stays solid.
When drug research groups choose Lark, they make a safe place where following the rules always comes first. This helps them keep up with the speed that patients and towns need.
